What is MPS VII?
MPS VII, known as Sly disease, is one of the mucopolysaccharide storage diseases. MPS VII was first identified by Dr Sly in 1972 and includes a spectrum of symptoms from mild to severe.
Mucopolysaccharides are long chains of sugar molecules used in the building of bones, cartilage, skin, tendons and many other tissues in the body. In the course of normal life there is a continuous recycling process of building new mucopolysaccharides and breaking down old ones. The breakdown and recycling process requires a series of special biochemical tools called enzymes.
People with MPS VII are missing or are low in an enzyme called beta-glucuronidase which is essential in breaking down 3 mucopolysaccharides dermatan sulphate, heparan sulphate and chondroitin sulphate.
When mucopolysaccharides are not completely broken down they remain stored in the body. The symptoms of MPS VII are a result of the build-up of dermatan sulphate, heparan sulphate and chondroitin sulphate in the tissues in the body.

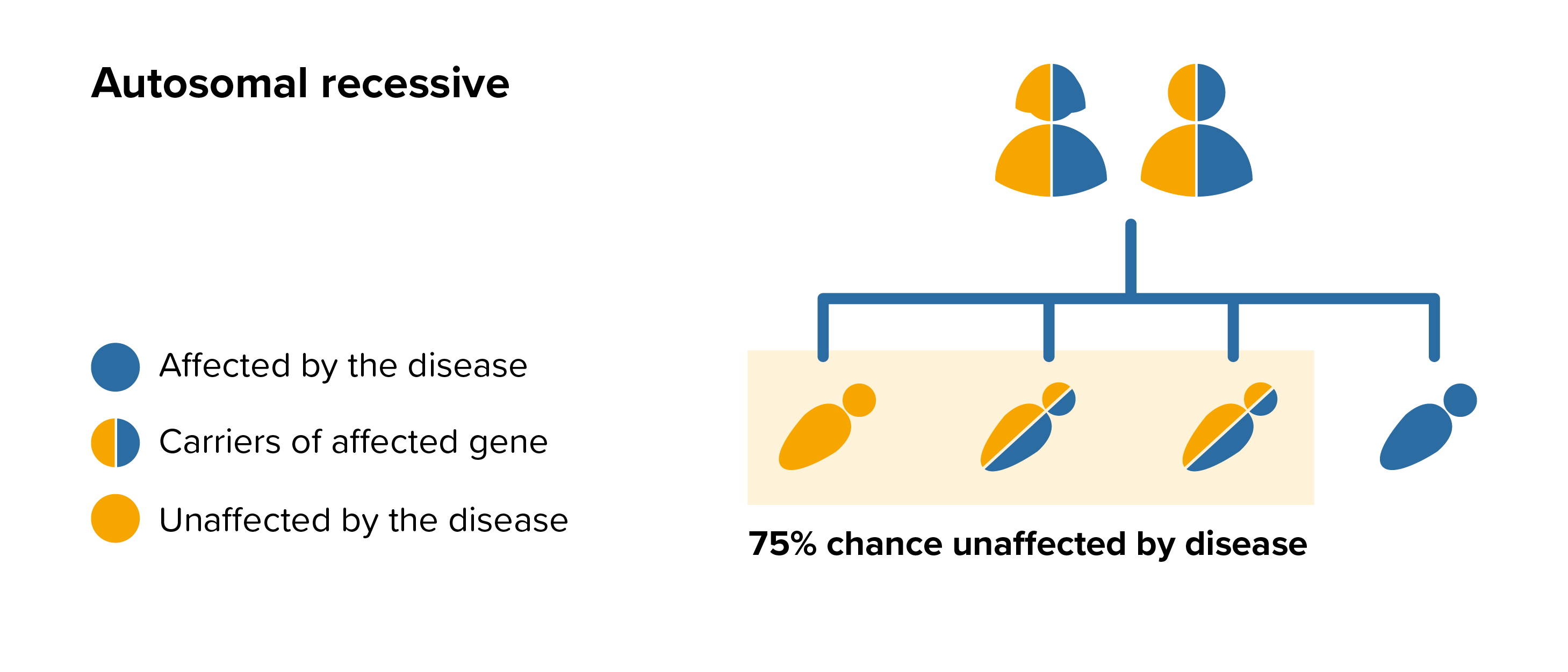 All parents of children with MPS VII can benefit from genetic counselling, the counsellor can provide advice on the risk to close relatives and to suggest whether the wider family should be informed. To find out during a pregnancy, if the baby is affected by MPS VII, screening tests can be arranged early on during a pregnancy for those families who already have a child with MPS VII. Where only one parent is a carrier, they can opt for carrier screening but it is not 100% reliable or accurate and is not possible in all cases.
All parents of children with MPS VII can benefit from genetic counselling, the counsellor can provide advice on the risk to close relatives and to suggest whether the wider family should be informed. To find out during a pregnancy, if the baby is affected by MPS VII, screening tests can be arranged early on during a pregnancy for those families who already have a child with MPS VII. Where only one parent is a carrier, they can opt for carrier screening but it is not 100% reliable or accurate and is not possible in all cases. 


